X-chromosome inactivation is a fascinating biological process that plays a crucial role in the genetics of female mammals. Unlike males, who possess only one X chromosome, females are equipped with two, necessitating the silencing of one copy to maintain genetic balance. This intricate mechanism is fundamental not only for normal cellular function but also in understanding genetic disorders such as Fragile X Syndrome and Rett Syndrome. Recent research led by Jeannie Lee at Harvard Medical School has illuminated the underlying processes of X-chromosome inactivation, offering promising avenues for gene therapy that could potentially alleviate the symptoms of these disorders. As chromosomal research continues to evolve, the implications of these findings pave the way for innovative treatments that could transform lives.
The phenomenon of X-chromosome silencing, often referred to as Lyonization, is essential for equalizing gene expression between the sexes in mammals. In this process, one of the two X chromosomes in females is purposefully inactivated to ensure that cellular functions remain balanced, preventing an excess of proteins encoded by X-linked genes. This regulation becomes particularly significant in the context of genetic conditions like Fragile X syndrome and Rett syndrome, where mutations can lead to severe developmental challenges. Scientists, including Jeannie Lee, are delving deep into the mechanisms of this chromosome regulation, revealing potential pathways for therapeutic interventions through gene therapy. By enhancing our understanding of chromosomal genetics, researchers are opening doors to cutting-edge treatments that could address the core issues of these debilitating conditions.
Understanding X-Chromosome Inactivation
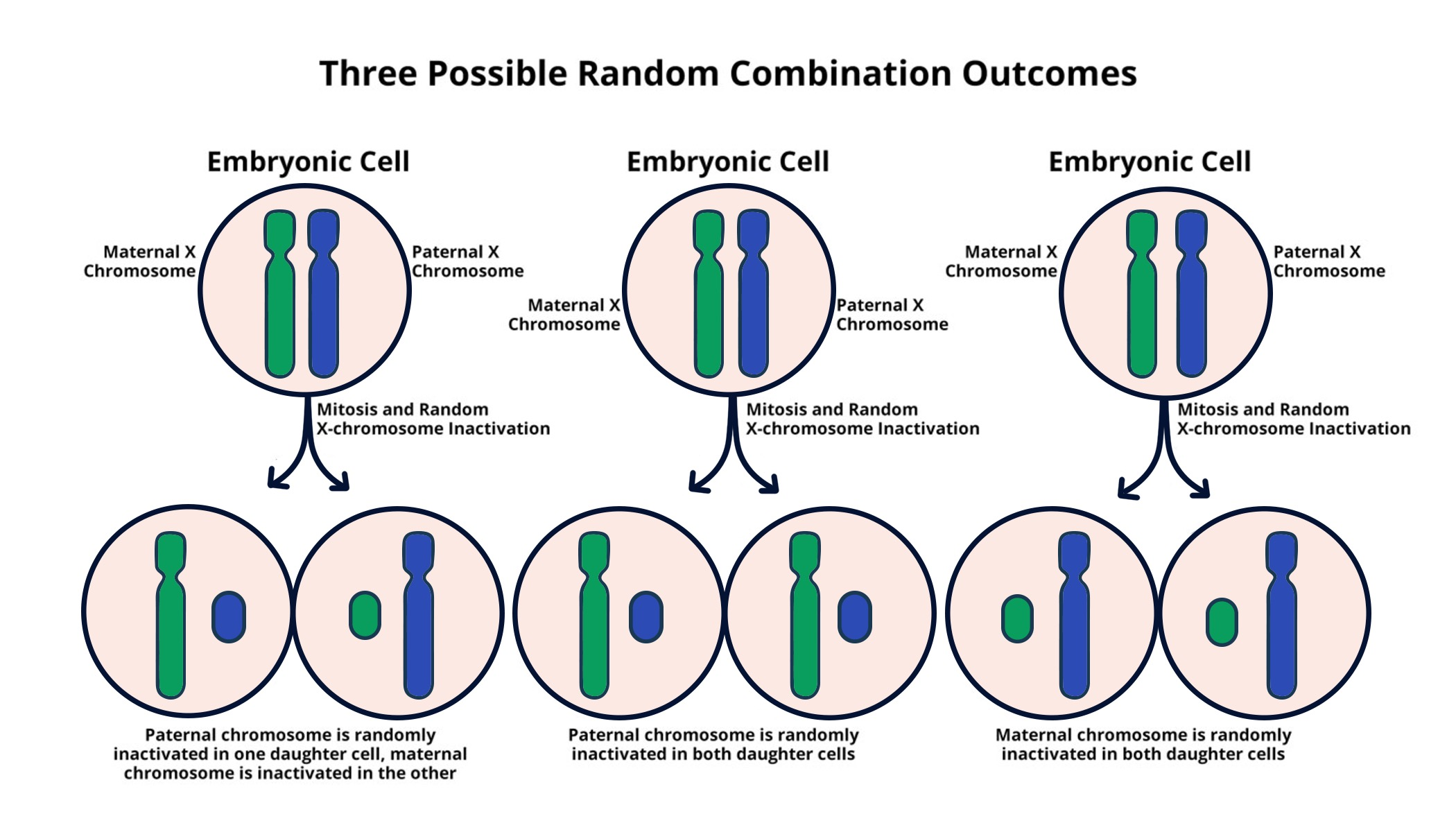
X-chromosome inactivation (XCI) is a crucial biological process that ensures gene expression balance in females, who possess two X chromosomes, as compared to males with one. This phenomenon is not merely a matter of gene dosage; rather, it is a sophisticated mechanism that regulates which X chromosome is expressed in each cell. Such regulation prevents excess production of X-linked gene products that could lead to cellular imbalance. Research led by Jeannie Lee has uncovered how this complex silencing occurs, revealing insights that may pave the way for innovative therapies for disorders linked to mutations on the X chromosome.
During X-inactivation, a specific gene called Xist plays a pivotal role by producing an RNA molecule that coats one of the X chromosomes, effectively silencing its genetic information. This process transforms the surrounding chromatin’s physical properties, much like the consistency of a Jell-O-like substance mentioned in recent studies. By understanding these mechanisms, researchers hope to leverage the principles of X-inactivation to develop targeted therapies for conditions such as Fragile X Syndrome and Rett Syndrome. The ultimate goal is to restore normal gene function in patients affected by these conditions.
The Promise of Gene Therapy for X-Linked Disorders
The promise of gene therapy offers a beacon of hope for individuals suffering from X-linked disorders such as Fragile X Syndrome and Rett Syndrome. These conditions are prevalent among individuals with mutations on the X chromosome, leading to severe cognitive and developmental challenges. Jeannie Lee’s research aims to utilize the mechanisms uncovered during the study of X-chromosome inactivation to find ways to unsilence the healthy genes that are often rendered inactive due to chromosomal architecture. This represents a significant leap forward in chromosomal research, as it introduces the potential for reversing the effects of these debilitating conditions.
In particular, gene therapy could provide a much-needed lifeline to those affected by Fragile X Syndrome, which is characterized by intellectual disabilities and emotional challenges. By understanding how to manipulate the Xist gene and the surrounding chromatin, researchers can design therapeutic strategies to unlock the potential of previously silenced genes. As a result, these approaches not only highlight the interplay between gene therapy and chromosomal research but also underscore the importance of continued investigations into the basic biology underlying X-chromosome inactivation.
The Role of Jeannie Lee’s Research in Chromosomal Breakthroughs
Jeannie Lee’s lab at Mass General has made significant strides in understanding chromosomal mechanisms, particularly X-inactivation. This research is essential for deciphering the complexities of how cells manage genetic information, especially in the context of diseases linked to X chromosome mutations, such as Fragile X Syndrome and Rett Syndrome. The team’s findings emphasize how a gelatinous substance surrounding chromosomes facilitates the process of inactivation, a discovery that sits at the intersection of fundamental biology and therapeutic innovation.
Moreover, Lee’s work stands as a testament to the long-term dedication required in chromosomal research. With over 25 years of investigation supported by the National Institutes of Health, her lab has transitioned from addressing fundamental scientific questions to exploring clinical applications. This journey illustrates the profound impact that basic research can have when translated into therapeutic avenues, offering hope not only for patients with specific genetic disorders but also for the broader field of genetic medicine.
Unlocking the Mysteries of Genetic Disorders
The mysteries surrounding genetic disorders like Fragile X and Rett syndrome highlight the intricate mechanisms at play when genetic information is expressed or silenced. In many cases, these disorders arise from mutations on the X chromosome, which complicates how cells manage these variations. Lee’s exploration into X-inactivation sheds light on why and how certain genes become inactive, providing insights that are essential for the development of effective treatments and interventions.
By focusing on the strategic manipulation of the X chromosome through techniques learned from her research on XCI, there is a significant opportunity to create targeted gene therapies that address the root causes of these disorders rather than merely managing their symptoms. The initial successes in untangling the complexities of chromosomal interactions not only enhance our understanding of developmental biology but also open new doors for clinical applications that can significantly improve patient outcomes.
Future Directions in Chromosomal Research
The future of chromosomal research looks promising, particularly as scientists like Jeannie Lee push the boundaries of what we know about X-chromosome inactivation and its applications in gene therapy. The anticipation surrounding forthcoming clinical trials is indicative of a growing field where innovative treatment options for Fragile X Syndrome and Rett Syndrome might soon become a reality. Researchers are exploring ways to optimize the approaches developed in the lab to ensure they are safe and effective for patients.
This forward momentum in chromosomal research not only highlights the importance of fundamental discoveries but also underscores the collaborative efforts required to translate basic science into viable clinical solutions. As scientific understanding deepens, researchers are likely to uncover more complex relationships between genetic mutations and cellular responses, paving the way for novel therapeutic strategies across multiple genetic disorders, ultimately transforming the landscape of medical treatment.
Implications of X-Chromosome Research on Genetic Disorders
The implications of ongoing research into X-chromosome inactivation extend far beyond Fragile X Syndrome and Rett Syndrome. As scientists delve deeper into the mechanisms regulating gene expression on the X chromosome, they are uncovering potential applications that could revolutionize treatments for a range of genetic disorders. The principal aim is to find ways to reactivate silenced genes, providing hope for patients whose conditions result from X-linked mutations.
Moreover, understanding X-inactivation contributes significantly to the broader scope of genetic research. It allows scientists to develop gene therapies tailored to reactivate specific genes while sparing healthy ones. This precision holds promise for minimizing side effects, which is typically a cornerstone concern when developing any new therapeutic approach. By using the insights gained from X-chromosome research, medical professionals can enhance treatment strategies and improve the quality of life for those affected by X-linked genetic syndromes.
Challenges Ahead in Developing Gene Therapies
Despite the exciting possibilities surrounding gene therapies derived from X-chromosome research, several challenges remain. First, significantly advancing from the lab bench to the clinic requires rigorous testing and validation of new approaches to ensure they are safe and effective for human use. The complexity of gene interactions, especially how they relate to X-linked disorders, presents ongoing challenges that researchers must navigate.
Furthermore, ethical considerations surrounding gene therapy also need to be addressed. The prospect of manipulating genetic material raises questions about long-term effects and the moral implications of altering the human genome. As researchers like Jeannie Lee make strides in the lab, balancing innovation with ethical responsibility will be key to moving forward with clinically applicable solutions for genetic disorders.
Collaborative Efforts in Genetic Research
Collaboration is essential in the domain of genetic research, where multifaceted challenges require input from various scientific disciplines. The interdisciplinary collaboration between geneticists, molecular biologists, and clinical researchers amplifies the pursuit of understanding complex disorders such as Fragile X and Rett Syndrome. This synergy can lead to innovative therapeutic strategies that are more effective and targeted.
Support from institutions such as the National Institutes of Health provides crucial funding and collaboration opportunities for researchers exploring the mechanisms behind X-chromosome inactivation. As these partnerships evolve, they enable the sharing of knowledge and resources, ultimately accelerating the pace of research and enhancing the potential for breakthroughs in treatments that can profoundly impact the lives of those suffering from X-linked genetic disorders.
The Intersection of Basic Science and Therapeutic Application
The intersection of basic science and therapeutic application is where much of the transformative power of genetic research lies. Understanding the fundamental biological processes, such as X-chromosome inactivation, lays the groundwork for developing therapies that can change lives, particularly for individuals with genetic disorders. The Lee lab exemplifies how answering basic biological questions can lead to tangible clinical applications.
As researchers continue to unravel the complexities of gene regulation, it becomes increasingly clear that foundational research is crucial for advancing medical science. The insights gained regarding X-chromosome inactivation not only enhance our comprehension of basic cellular processes but also illuminate new pathways to developing effective gene therapies. This relationship illustrates the vital importance of fostering curiosity-driven research as it has the potential to translate directly into therapeutic breakthroughs for a variety of genetic conditions.
Frequently Asked Questions
What is X-chromosome inactivation and its significance in genetics?
X-chromosome inactivation is a biological process where one of the two X chromosomes in female mammals is randomly inactivated, preventing dosage imbalance of X-linked genes. This process is crucial for understanding genetic diseases, particularly those linked to mutations on the X chromosome, such as Fragile X Syndrome and Rett Syndrome.
How does X-chromosome inactivation relate to Fragile X Syndrome and Rett Syndrome?
X-chromosome inactivation plays a pivotal role in the manifestations of Fragile X Syndrome and Rett Syndrome, both of which involve mutations on the X chromosome. While female carriers may inactivate the mutated X, the active, unaffected X can potentially compensate for these mutations, making the understanding of this mechanism essential for developing gene therapies.
Who is Jeannie Lee and what is her contribution to the study of X-chromosome inactivation?
Jeannie Lee is a prominent researcher at Harvard Medical School known for her groundbreaking work on X-chromosome inactivation. Her lab has provided critical insights into how the inactivation process occurs and its implications for treating X-linked genetic disorders like Fragile X Syndrome and Rett Syndrome.
What recent advancements have been made in chromosomal research regarding X-chromosome inactivation?
Recent advancements in chromosomal research have revealed that a gelatinous substance surrounding chromosomes plays a crucial role in X-chromosome inactivation. Understanding this process opens pathways for potential gene therapy treatments for conditions like Fragile X Syndrome and Rett Syndrome by enabling researchers to unsilence inactivated genes.
What role does gene therapy play in addressing issues related to X-chromosome inactivation?
Gene therapy is positioned to address the challenges associated with X-chromosome inactivation by providing methods to unsilence mutated genes found on the inactivated X chromosome. This approach holds promise for treating X-linked disorders such as Fragile X Syndrome and Rett Syndrome by restoring the function of affected genes.
Why is it important to understand the mechanisms of X-chromosome inactivation in gene therapy development?
Understanding the mechanisms of X-chromosome inactivation is critical in gene therapy development because it allows researchers to explore ways to reactivate silenced genes. By focusing on X-linked diseases like Fragile X Syndrome and Rett Syndrome, therapies can be designed that minimize side effects, targeting only the mutated genes while preserving the function of healthy ones.
| Key Points |
|---|
| Females have two X chromosomes, while males have one; one X in females undergoes inactivation to balance gene dosage. |
| Jeannie Lee’s research focuses on how cells silence one X chromosome, using a gelatinous substance known as Jell-O to facilitate this process. |
| The gene Xist plays a critical role in modifying the Jell-O around the X chromosome, allowing for proper inactivation. |
| Uncovering X-chromosome inactivation has potential therapeutic implications for diseases like Fragile X Syndrome and Rett Syndrome, leading to innovative treatments. |
| Research indicates that freeing inactivated X chromosomes may enhance the functionality of mutated genes but leave healthy genes largely unaffected. |
| The work of Lee’s lab has evolved from basic research to potential clinical applications, highlighting the long journey of scientific discovery. |
Summary
X-chromosome inactivation is a crucial biological process essential for balancing gene expression in females. This phenomenon helps prevent gene dosage imbalances by silencing one of the two X chromosomes. The research led by Jeannie Lee at Harvard Medical School has unveiled the intricate mechanisms behind this process, particularly emphasizing the role of Xist and a gelatinous substance that facilitates chromosome organization. As advances continue in understanding X-chromosome inactivation, promising therapeutic strategies are emerging for genetic disorders linked to mutations on the X chromosome, including Fragile X Syndrome and Rett Syndrome. The future of this research could lead to effective treatments that impact countless patients.